
There is a growing need to refocus efforts on how diversity is achieved in clinical trials, because some are doing it wrong despite their best intentions.

There is a growing need to refocus efforts on how diversity is achieved in clinical trials, because some are doing it wrong despite their best intentions.

Some actions to consider for your next trial or submission.
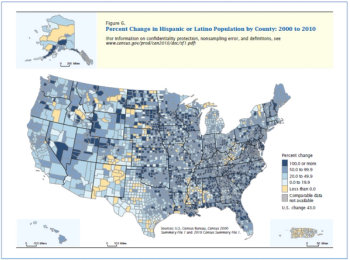
It should suffice to say that obtaining informed consent is required ethically and legally in almost all forms of human research in almost all countries.
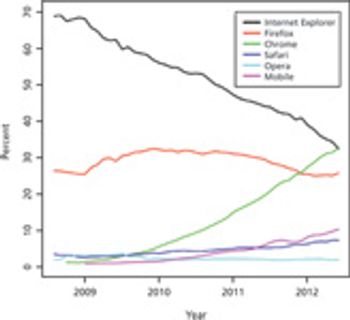
Options have emerged that make DIY EDC technology more accessible to smaller organizations.

While risk and uncertainty remain high, the pace of innovation will remain glacial.

An Applied Clinical Trials Editorial Advisory Member gives his opinion on where the differences lie between EDC and ePRO systems.

Some starry-eyed vendors can't turn their back on older technology because they're too attached.
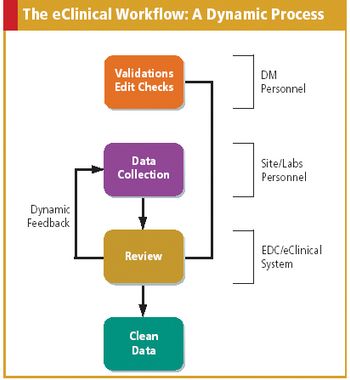
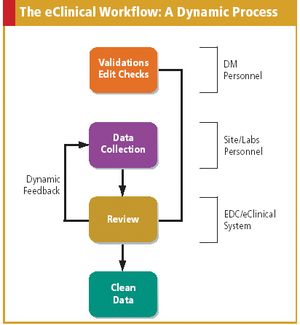
As more companies adopt eClinical technologies, data managers must redefine and update their role.

A story about 1/2-inch holes and the power of liquid soap

Clinical departments are very expensive to run and maintain.
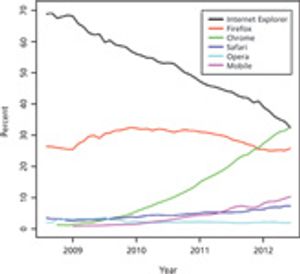
Published: February 1st 2013 | Updated:

Published: January 1st 2012 | Updated:

Published: June 16th 2009 | Updated:

Published: June 1st 2007 | Updated:
Published: October 1st 2006 | Updated:

Published: March 1st 2006 | Updated: