
Applied Clinical Trials
EMEA and EC admit to legislation difficulties, and many voice their ideas in an effort to make improvements.

Applied Clinical Trials
EMEA and EC admit to legislation difficulties, and many voice their ideas in an effort to make improvements.
Applied Clinical Trials
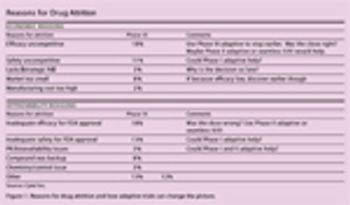
No longer a futuristic concept, adaptive clinical trials have become a reality that must be considered.

Applied Clinical Trials

Applied Clinical Trials
FDA and sponsors implement FDAAA as pressure builds to curb drug prices and tweak the R&D process.

Applied Clinical Trials
TrialStat's EDC Platform Adds Graphical Reporting Features and More

Applied Clinical Trials
Steady enrollment and optimal trial metrics can become reality with the right processes and tools.

Applied Clinical Trials
Lack of minority clinical investigators behind dearth of minority subjects in trials.
Applied Clinical Trials
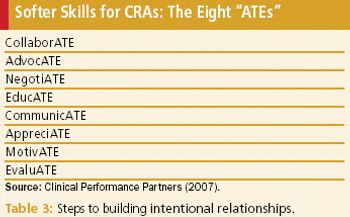
Both parties benefit from intentional friendships, and nowadays CRAs are key to this cordial effort.

Applied Clinical Trials

Applied Clinical Trials
Recent announcements streamline adverse event reporting

Applied Clinical Trials
Saul Shiffman, cofounder and chief scientific officer of invivodata, Inc., examines how patient-reported data collection has been transformed since 1987.