
Applied Clinical Trials
Outlining the growing attention and pursuits around social media in adverse event reporting and advancing patient-centric initiatives in clinical trials.

Applied Clinical Trials
Outlining the growing attention and pursuits around social media in adverse event reporting and advancing patient-centric initiatives in clinical trials.

Applied Clinical Trials
Now the challenge to FDA and to sponsors is to maintain the high level of support for research, discovery, and regulatory flexibility underpinning these gains, writes Jill Wechsler.

Applied Clinical Trials
Peter O’Donnell looks at efforts in Europe to improve R&D communication and trust with investors and the public.

Applied Clinical Trials
An expert view on how sponsors can formalize the use of real-world data and generation of real-world evidence to drive critical insights.
Applied Clinical Trials
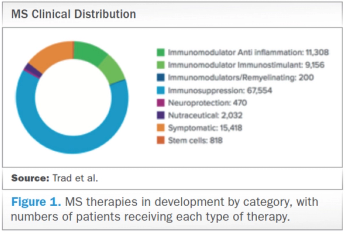
Examining the main challenges in designing and executing MS clinical trials and proposing mitigation strategies that may help alleviate these burdens.

Applied Clinical Trials
To vie for success in the market for dermatologic therapies, companies developing biologics must navigate a series of significant challenges, including patient compliance and safety.
Applied Clinical Trials
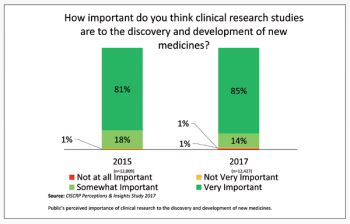
The first series of results from the Center for Information and Study on Clinical Research Participation’s (CISCRP) landmark 2017 Perceptions & Insights Study.

Applied Clinical Trials
Findings from a new ACT and SCORR Marketing survey reveal a gap in the shift to true patient-engagement in clinical trials, but overall measures do signal growth in patient-centric activities.

Applied Clinical Trials
Lisa Henderson reports on the J.P. Morgan Healthcare conference and the balance of investment and science.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials January/February 2018 issue in an interactive PDF format.