Applied Clinical Trials
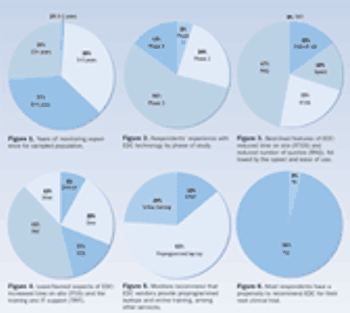
For years, EDC providers have claimed that the technology can increase efficiency and potential cost savings. Survey results indicate that clinical monitors agree.

Applied Clinical Trials
For years, EDC providers have claimed that the technology can increase efficiency and potential cost savings. Survey results indicate that clinical monitors agree.
Applied Clinical Trials
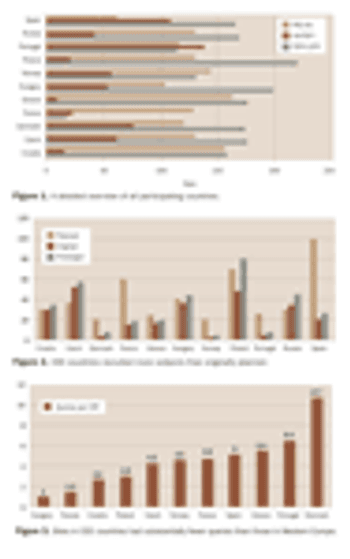
Pharmaceutical companies and contract research organizations are setting up an increasing number of clinical trials in Central and Eastern Europe. The region offers attractive opportunities for sponsors to accelerate subject recruitment and to carry out clinical trials according to harmonized international standards and regulations. Recent concerns about data quality and cost-efficiency at CEE investigational sites, however, have led to regular evaluation of sites' clinical research performance and capacity. Now it is essential to introduce benchmarking and performance measurement to the conduct of clinical trials to identify best practices and to continually improve clinical research.

Applied Clinical Trials
Improving the informed consent process and consent forms can not only help subjects better understand clinical research but also reduce the number of invalid consents obtained.

Applied Clinical Trials
HHS offers IRBs, institutions, and investigators points to consider when dealing with financial relationships and conflicts of interest in clinical research.

Applied Clinical Trials
Postmarketing research is conducted differently than preapproval research, but data gained using different standards can be difficult to integrate.

Applied Clinical Trials
A newly completed study of European ethics committees finds that the bureaucracy of each country determines the way the ethics committees operate.
