
Applied Clinical Trials
Protecting Subjects: The IRBs Next Steps

Applied Clinical Trials
Protecting Subjects: The IRBs Next Steps

Applied Clinical Trials
SITES : Incorporating Standard of Care in Study Budgets Benefits of CTMS at the Investigative Site Also in this issue : FDA Transparency Efforts to Impact Research, Survey Assesses Europe?s Clinical Trials Directive, Tapping into the Potential of Pharmacists, Biosimilars Make Headway in the U.S.

Applied Clinical Trials
Updates on Osteoporosis including Amgen and Prolia trials.


Applied Clinical Trials
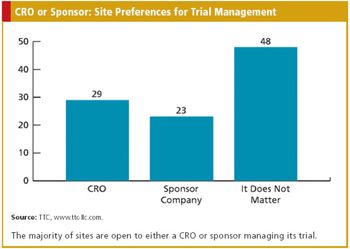
Do Sites Prefer a Sponsor Company or CRO to Run Their Clinical Trial?
Applied Clinical Trials
An overview of the clinical research landscape in this emerging region that also looks at its challenges.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
The verdict is awaited on the much criticized and long anticipated European clinical trials directive.

Applied Clinical Trials
Survey reveals industry practices surrounding standard of care and insurance claims data.

Applied Clinical Trials
The 12-year data exclusivity period has significant implications in delaying the launch of biosimilars.

Applied Clinical Trials
More information may be available on drug applications to expand public understanding of FDA policies.

Applied Clinical Trials
A recent survey indicates pharmacists should provide more clinical trial information to patients.

Applied Clinical Trials
Clinical trial management systems seamlessly support enhanced financial productivity for sites.