
Applied Clinical Trials
2010 Directory & Buyers Guide Services & Products Company Index

Applied Clinical Trials
2010 Directory & Buyers Guide Services & Products Company Index

Applied Clinical Trials
CRO/SPONSOR : The Nuances of Medical Device Trials A Triggered Approach to Site Monitoring INFORMATION TECHNOLOGY : Automating the Phase I Trial Also in this issue : FDA Addresses Concerns Over Foreign Studies, The Cost Factor in EU Drug Authorization, Baseball?s Ties to Comparative Effectiveness Research, Key Updates to the Form FDA 1572

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
How to make the leap beyond paper and EDC to create an automated clinical environment that thrives.

Applied Clinical Trials
FDA is saying that a study coordinator "generally" performs critical functions, such as subject recruitment.
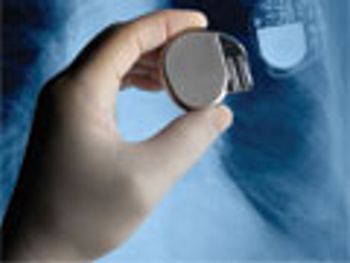
Applied Clinical Trials
On time and on budget are always necessary for a clinical trial, but medical devices offer nuances.

Applied Clinical Trials
Among the many clinical development processes that need to be conducted in a smarter, more cost-effective manner, clinical data monitoring stands out as a promising area in which operational efficiencies can not only reduce costs but also improve research quality and patient safety.

Applied Clinical Trials
Inspector General study focuses attention on quality of data and patient safeguards.

Applied Clinical Trials
European network could bring confusion to health technology assessment and clinical research.


Applied Clinical Trials
The importance of comparative effectiveness research and how to overcome its challenges.


Applied Clinical Trials
The European Medicines Agency has redesigned its Web site to improve transparency.
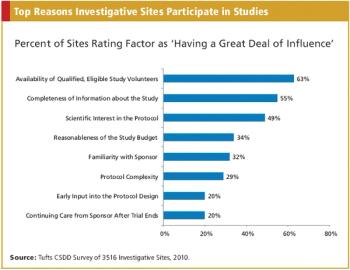
Applied Clinical Trials
The design and completeness of the protocol and early and open communication with research center personnel are factors that most influence investigative site willingness to participate in a clinical study.