
Applied Clinical Trials
The rush is on to develop new therapies and vaccines to combat the lethal outbreak.

Applied Clinical Trials
The rush is on to develop new therapies and vaccines to combat the lethal outbreak.

Applied Clinical Trials
Research shows that the potential of integrated alliances remains elusive in the near term.

Applied Clinical Trials
The technology necessary to meet the expected regulatory demands for assessing drug-impaired driving has emerged.

Applied Clinical Trials
Reshuffling to heavily impact life sciences policy in Europe.

Applied Clinical Trials
A Medical Technology Working Party is established to promote collaboration and dialogue on the specific aspects of clinical standards.

Applied Clinical Trials
Exploring sourcing model options for appropriate incorporation of DMCs into a clinical program.
Applied Clinical Trials
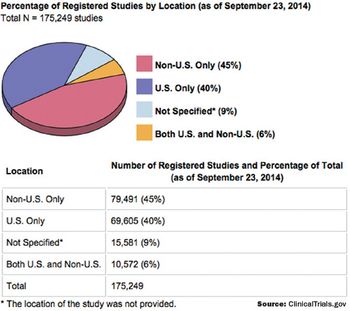
The distribution of locations for all trials registered on ClinicalTrials.gov.


Applied Clinical Trials
Proving a biosimilar's pharmacokinetic 'equivalence' requires adherence to several unique factors.

Applied Clinical Trials
A systematic approach for early identification of BP effects during development of new drugs.

Applied Clinical Trials
Trial Design: Identify Blood Pressure Effects Early Demonstrating Biosimilarity CRO/Sponsor: Data Monitoring Committee Users Guide Also in this issue: EU Reshuffling Impacts Pharma "Open Integration" Promise Elusive Assessing Drug-Impaired Driving