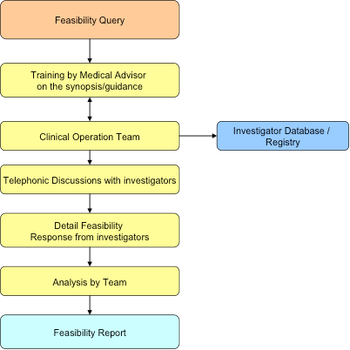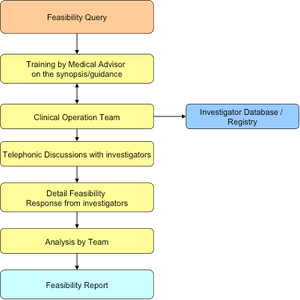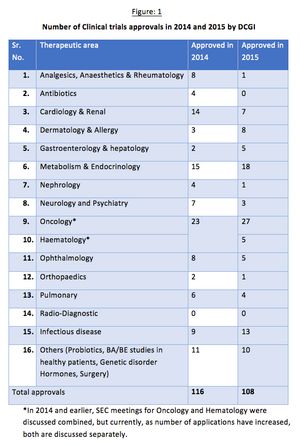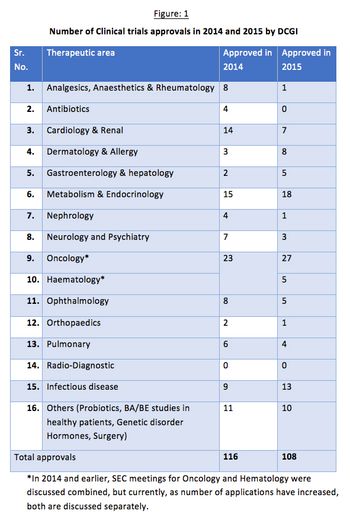
Issues concerning the drug approval process in India have occurred in clinical studies resulting in a reduced interest from sponsors. The Drug Controller General of India (DCGI) has been working to amend and update it’s policies to stimulate growth in this sector.