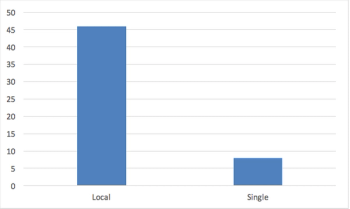
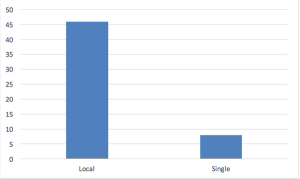
Since the emergence of the independent IRB sector, sponsors have found that regulatory and ethical review by a single IRB yields important benefits with respect to efficiency, high quality, and consistency in human research protections.
Published: September 20th 2017 | Updated: