
Outlining the techniques for anonymization of clinical study reports and the identification and redaction of commercially confidential information to comply with EMA's Policy 0070 on trial data disclosure and transparency.

Outlining the techniques for anonymization of clinical study reports and the identification and redaction of commercially confidential information to comply with EMA's Policy 0070 on trial data disclosure and transparency.
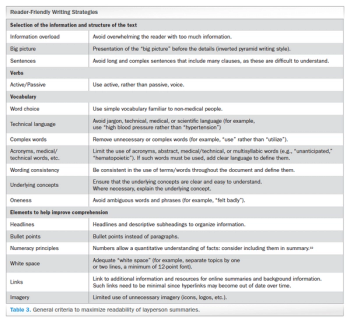
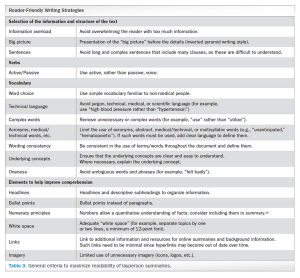
Analyzing the debate around writing and constructing layperson summaries of clinical trial results-and proposing a “reader-centered” approach.

Outlining considerations on layperson summary writing of clinical trial results in Europe, and proposing a "reader-centered" approach to constructing these summaries.

In 2014, EU Regulation n.536/2014 represented a significant step toward transparency of clinical trials in Europe. However, despite the EMA’s policy 0070, which reinforced this concept, several aspects remain to be determined or evaluated, leaving room for additional requirements and local interpretation.

Published: November 3rd 2016 | Updated:

Published: July 11th 2017 | Updated:
Published: August 1st 2017 | Updated:

Published: December 18th 2017 | Updated: