
With the U.K. poised to begin the process of leaving the European Union soon, many questions remain as to the specifics of pharma regulation and the wide-ranging effects of this departure.

With the U.K. poised to begin the process of leaving the European Union soon, many questions remain as to the specifics of pharma regulation and the wide-ranging effects of this departure.

Following the UK’s decision to leave the European Union, the result has been uncertainty and speculation as pharma attempts to understand its implications. How this change will effect the pharma and life sciences industry as well as the European community remains to be seen.

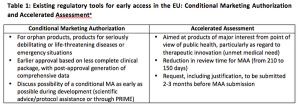
In response to the debate over allowing patients timely access to new therapies and ensuring safety, the EMA launched the PRIority MEdicines (PRIME) scheme in March. The aim of this initiative is to build upon existing regulations in Europe to support product development in cases of unmet medical need.
Published: June 13th 2016 | Updated:

Published: September 6th 2016 | Updated:

Published: February 28th 2017 | Updated: