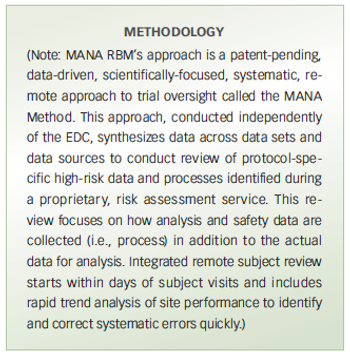
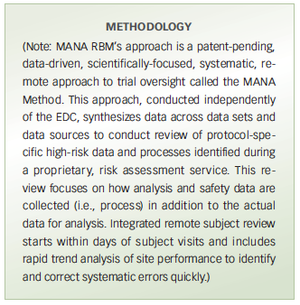
Pilot study compares a risk-based monitoring and remote trial management method with traditional on-site source data verification for trial oversight.

Pilot study compares a risk-based monitoring and remote trial management method with traditional on-site source data verification for trial oversight.

A new RBM method used in PaxVax trial proves successful vs. onsite source data verification for trial oversight.

Published: September 28th 2018 | Updated:
Published: November 1st 2018 | Updated: