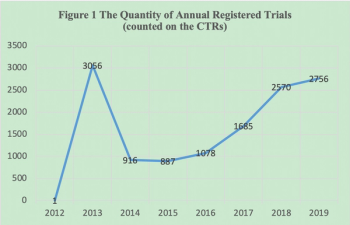
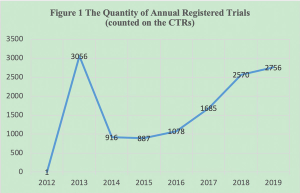
Following the implement of new Drug Registration Regulation in July 2020, the clinical trial registration system will have some major changes in China.

Following the implement of new Drug Registration Regulation in July 2020, the clinical trial registration system will have some major changes in China.
Published: May 20th 2020 | Updated: