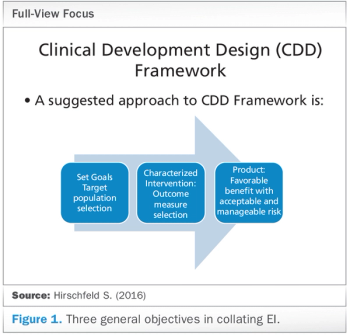
How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."

How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."
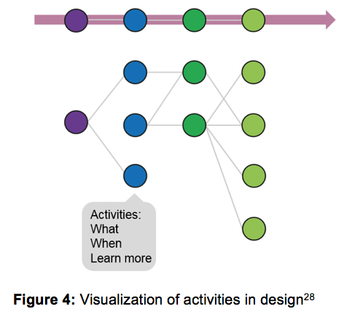
How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."
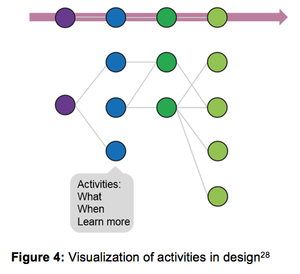
Published: November 9th 2017 | Updated:
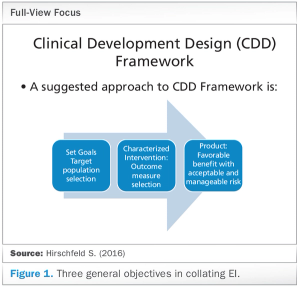
Published: April 1st 2018 | Updated: