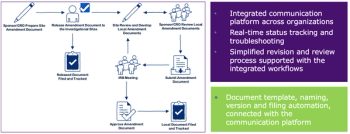
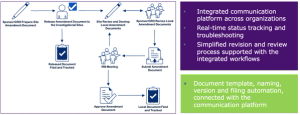
Trials that address multiple questions simultaneously using a master protocol can be operationally complicated. These complexities can be managed, even in studies used to support a marketing application.

Trials that address multiple questions simultaneously using a master protocol can be operationally complicated. These complexities can be managed, even in studies used to support a marketing application.
Published: January 29th 2021 | Updated: