
The general assumption that the Policy 0070 data releases were public data releases can be challenged since there is a mechanism to enforce the ToU, and this mechanism will continue to exist after the Agency moves to the Netherlands.

The general assumption that the Policy 0070 data releases were public data releases can be challenged since there is a mechanism to enforce the ToU, and this mechanism will continue to exist after the Agency moves to the Netherlands.
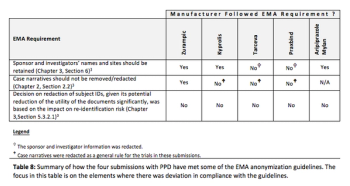
Examining early learnings from approaches used to comply with EMA’s requirement to publish anonymized versions of clinical study reports and other submission documents, including how privacy protection was balanced against data utility.

Whether we are referring to CSRs or to IPD, the personal information of trial participants needs to be de-identified prior to release. This article will describe the available methods for de-identifying clinical trial data, and the relative strengths and weaknesses of each.

Whether we are referring to CSRs or to IPD, the personal information of trial participants needs to be de-identified prior to release. This article will describe the available methods for de-identifying clinical trial data, and the relative strengths and weaknesses of each.

Published: March 20th 2015 | Updated:

Published: August 1st 2015 | Updated:
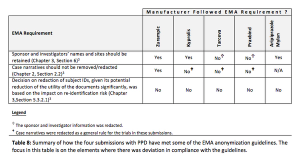
Published: April 13th 2017 | Updated:

Published: June 25th 2018 | Updated: