
Leveraging approaches in RBQM to enable effective corrective and preventive action processes.

Leveraging approaches in RBQM to enable effective corrective and preventive action processes.
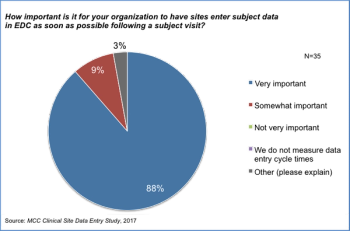
With recent reports indicating that organizations do not have a way of tracking and managing the timeliness of data entry or of site payments, The Metrics Champion Consortium wanted to see if there were opportunities to align incentives in the industry so that both sites and sponsors can achieve their goals with positive impact on clinical trials and patients.

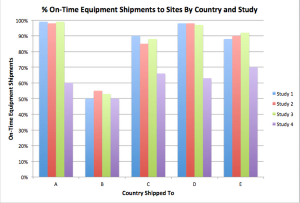
The pharma industry agrees that the importance of entering data into an EDC as soon as possible following a subject is paramount. Slow site data entry can impact the credibility and usefulness of centralized monitoring data analytic reports.
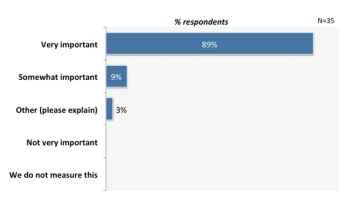
The pharma industry agrees that the importance of entering data into an EDC as soon as possible following a subject is paramount. Slow site data entry can impact the credibility and usefulness of centralized monitoring data analytic reports.

Just because information can be gathered and shared more quickly among stakeholders does not mean that it can identify risk. This article describes the need for pharma to adopt fully-vetted, standardized operational-level time, cost and quality performance metrics as tools for tracking and predicting performance.
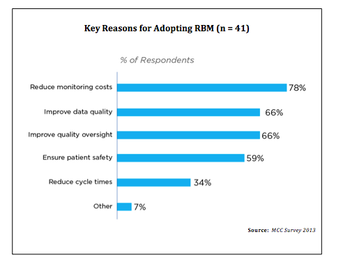
A comprehensive survey by Metrics Champion Consortium

Central laboratory analysis of samples collected by the investigational sites is an important source of safety and efficacy endpoint data.
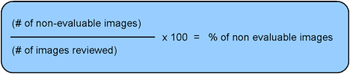
Medical imaging is an important source of subject eligibility, drug efficacy, and safety data for many clinical trials.
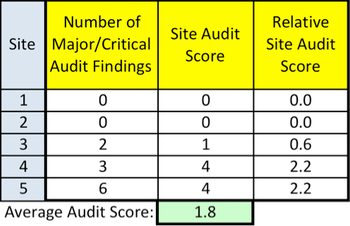
MCC has just published an executive summary of its Risk Based Monitoring Usage Survey.
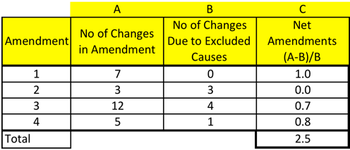
This month, let's look at a quality metric that's critical to on-time, on-budget performance: protocol amendments.

This month, let's look at a quality metric that's useful for tracking both protocol and site performance: subject retention percentage.

MCC has just published an executive summary of its Risk Based Monitoring Usage Survey
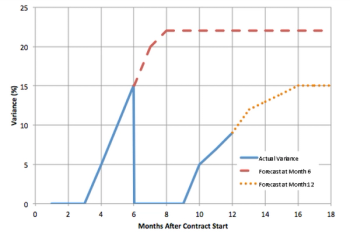
Let's look at a valuable cost metric: percent variance from budget.
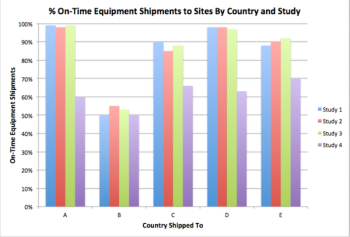
MCC has 100 different metrics relating to clinical trials, from timeliness and cycle time metrics to quality, efficiency, and cost metrics.
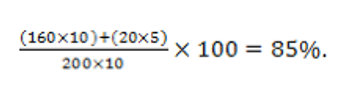
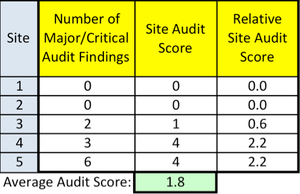
Quality metrics are hard to define and measure, but are crucially important.
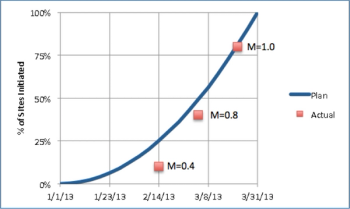
We'll start our monthly metrics blog with a basic but crucial MCC Clinical Trial Performance metric: on-time site initiation.

That's one of our key aphorisms here at the Metrics Champion Consortium (MCC). We passionately believe that measurement can effect change, and we?ve spent the last eight years working with our members to define the best metrics for tracking performance and improvement in the industry.
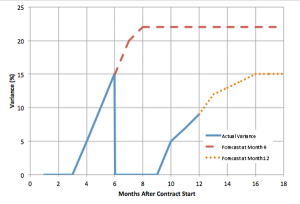
Published: March 6th 2014 | Updated:
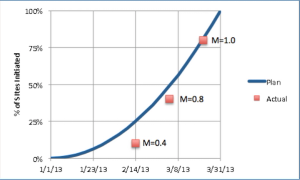
Published: November 15th 2013 | Updated:
Published: January 17th 2014 | Updated:
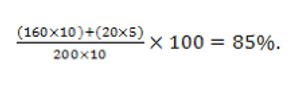
Published: December 16th 2013 | Updated:

Published: October 28th 2013 | Updated:
Published: July 21st 2014 | Updated: