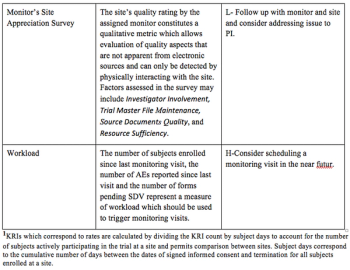
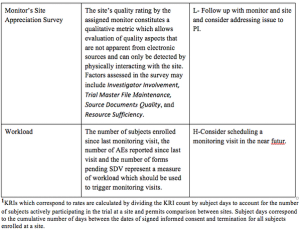
Pilot study evaluates the success of building quality into clinical trials using a plan-do-check-act (PDCA) approach to quality management.

Pilot study evaluates the success of building quality into clinical trials using a plan-do-check-act (PDCA) approach to quality management.
Published: October 26th 2015 | Updated: