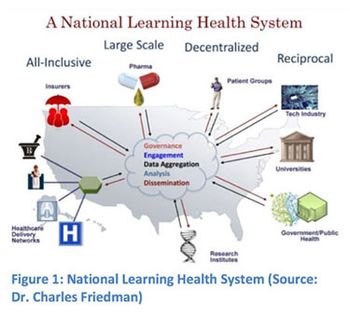
Outlining the ways FDA is adopting electronic practices and initiating efforts to simplify the submission process for new drug and generic drug applications.
Mary Ann Slack is Deputy Director, Office of Strategic Programs, Center for Drug Evaluation and Research (CDER), Food & Drug Administration.
Published: August 1st 2017 | Updated: