
Outlining the unique pharmacokinetic factors that should be considered when designing and running early stage clinical trials for monoclonal antibodies.

Outlining the unique pharmacokinetic factors that should be considered when designing and running early stage clinical trials for monoclonal antibodies.

There are several pharmacokinetic factors and peculiarities that should be considered carefully when designing and running first-in-human (FIH) studies for monoclonal antibodies.
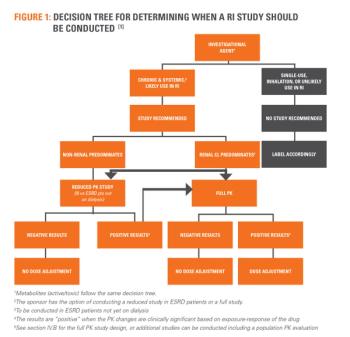
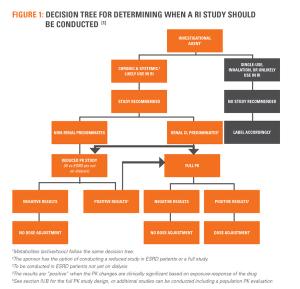
Studies in Hepatic and Renal Impaired special populations have almost always required drugs with systematic absorption resulting in unclear conclusions. A solution for this would be a study design based on pharmokinetic properties that incorporates principles of Hepatic and Renal Impaired pathologies.
Published: August 26th 2016 | Updated:

Published: May 4th 2018 | Updated:

Published: June 1st 2018 | Updated: