
Case study demonstrates how one global mid-sized sponsor is testing the RBM waters.

Case study demonstrates how one global mid-sized sponsor is testing the RBM waters.
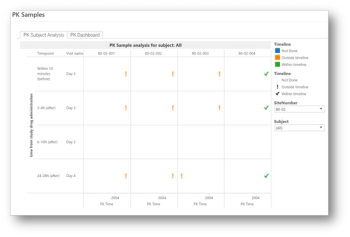
Risk-based approaches to monitoring (RBM) have become widely accepted in the clinical trials industry.
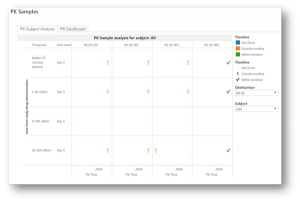
Published: December 17th 2018 | Updated:

Published: January 1st 2019 | Updated: