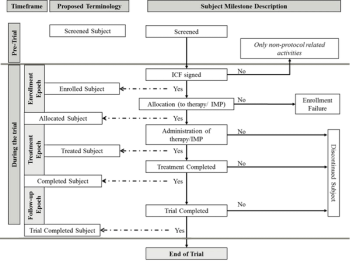
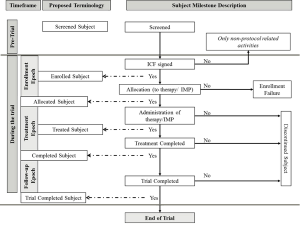
Subject disposition, which is the flow of patients through a clinical trial, requires further discussion for harmonization of terms.

Subject disposition, which is the flow of patients through a clinical trial, requires further discussion for harmonization of terms.
Published: February 13th 2015 | Updated: