
During an audit, the FDA investigates six areas to determine whether a site is in compliance with federal drug accountability regulations-can sponsors answer them?

During an audit, the FDA investigates six areas to determine whether a site is in compliance with federal drug accountability regulations-can sponsors answer them?
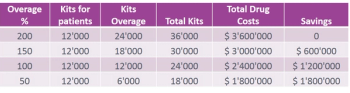
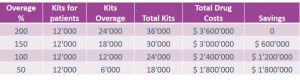
Managing clinical trial supply is not easy for the pharmaceutical industry between drug overages and right medical distribution. However, this is an important topic for sponsors to consider because the right strategy can help save money and reduce unnecessary spending.
Published: September 14th 2017 | Updated:

Published: June 1st 2018 | Updated: