
Applied Clinical Trials
Regulation will benefit both children & drug development, says EMEA, which has a lot on its plate this year.

Applied Clinical Trials
Regulation will benefit both children & drug development, says EMEA, which has a lot on its plate this year.

Applied Clinical Trials
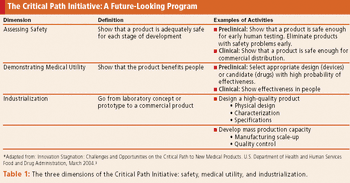
Drug safety concerns, unproductive methods, and high costs encourage innovative study designs and collaborative efforts.

Applied Clinical Trials
The fate of a new bird flu vaccine that provides limited protection rests in the hands of FDA.
Applied Clinical Trials
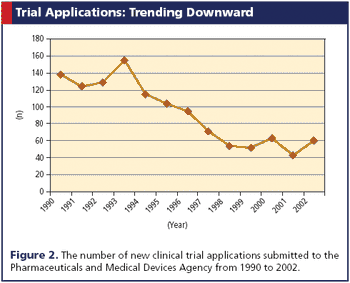
Launched in 2003, the LCN Project calls for more studies and an improved trial infrastructure.
Applied Clinical Trials
Outsourcing and trial management tool puts professionals in control of all aspects of a study
Applied Clinical Trials
Digital writing solution speeds up data harvest while ensuring traceability and capture ease
Applied Clinical Trials
Interaction is high on FDA's list of ways to improve the development & review process for technologies.

Applied Clinical Trials
Virtualization offers companies a practical solution to bypass server limitations.

Applied Clinical Trials
Dual-purpose package allows submission in both paper and electronic formats
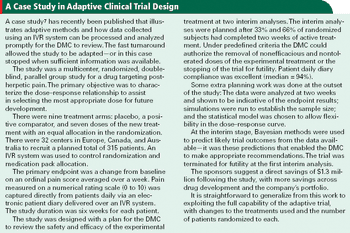
Applied Clinical Trials
Interactive voice response systems are a good match for adaptive clinical trials and can help keep investigators in the dark.
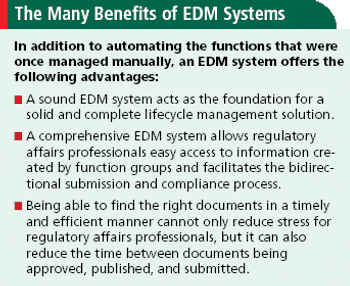
Applied Clinical Trials
Why an electronic document management system is key to getting bought by major pharma.