
Applied Clinical Trials
Recent initiatives demonstrate that European health authorities are serious about combatting the proliferation of threats and opportunities from big data.

Applied Clinical Trials
Recent initiatives demonstrate that European health authorities are serious about combatting the proliferation of threats and opportunities from big data.

Applied Clinical Trials
Survey uncovers deeper learnings of patient perceptions of clinical research and the motivations to participate.

Applied Clinical Trials
FDA officials provide more specific plans for streamlining and modernizing plans for assessing and regulating products for cutting-edge cellular and gene-based medical treatments.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials March 2019 issue in an interactive PDF format.

Applied Clinical Trials
Patient advocacy organizations are swaying away from a traditional model of granting academic researchers to now supporting biotechnology and pharma companies.
Applied Clinical Trials
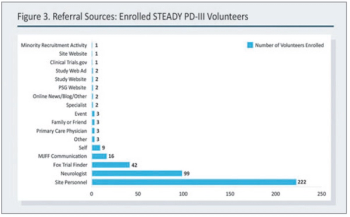
A case study of a Parkinson’s disease trial demonstrates how one study team met its enrollment goals.

Applied Clinical Trials
Patient advocacy groups, patient centricity initiatives, and patient education are the key to putting patients at the center of clinical research.

Applied Clinical Trials
Tracking the evolution and effectiveness of CISCRP’s clinical research awareness and literacy campaigns.

Applied Clinical Trials
In this Q&A, Helen Matthews, Jessica Morris, and Bruce Hellman offer their perspectives on the priorities, opportunities, and challenges of patient centricity in real-world evidence collection.

Applied Clinical Trials
Optimization model evaluates the benefits of selecting a portfolio of investigative sites based on advanced analytical models.

Applied Clinical Trials
Examining the distinct actions and advocacy that have advanced the concept from buzzword status to practical implementation in clinical studies.

Applied Clinical Trials
It’s time to establish standard practices to return clinical trial results summaries to patients.