Applied Clinical Trials
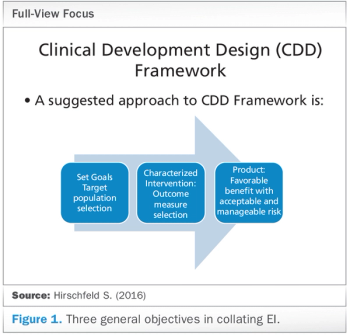
How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."

Applied Clinical Trials
How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."

Applied Clinical Trials
Debunking the six most common myths regarding electronic informed consent.

Applied Clinical Trials
In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.

Applied Clinical Trials
Jill Wechsler on the tug of war in accelerating orphan drug development.

Applied Clinical Trials
Relocating the European Medicines Agency was always going to be hard-but no-one ever expected it to degenerate into farce.
Applied Clinical Trials
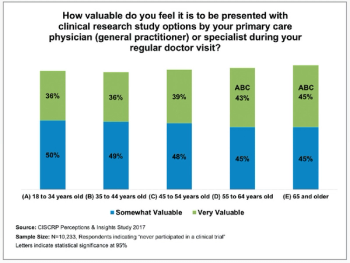
The second series of results from the Center for Information and Study on Clinical Research Participation’s (CISCRP) landmark 2017 Perceptions & Insights Study.

Applied Clinical Trials
The use of natural history (NH) studies early in clinical research can help facilitate development programs for orphan drugs.

Applied Clinical Trials
Lisa Henderson discusses randomized clinical trials with active and non-active placebo treatments.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials April 2018 issue in an interactive PDF format.