
Applied Clinical Trials
A new study from Tufts highlights employee and fiscal growth in the industry from 2002 to 2005.

Applied Clinical Trials
A new study from Tufts highlights employee and fiscal growth in the industry from 2002 to 2005.

Applied Clinical Trials
When it comes to readability, do as the Feds say and not as they do. Federal agencies may recommend that consent forms be written on a junior high school level, but it's a daunting task even for them.
Applied Clinical Trials
CTSD Version 2.5 provides improved data visualization and enhanced user interface

Applied Clinical Trials
India's investigators play a key role in helping CROs & sponsors recruit patients and meet global standards.

Applied Clinical Trials
Policy makers discuss incentives for trials on children and for generic versions of biotech therapies.
Applied Clinical Trials
Safety issues pinpointed earlier with Spotfire's interactive analysis data solution

Applied Clinical Trials
Strategies sponsors can employ to ensure vendors are complying with regulations-even when none exist.
Applied Clinical Trials
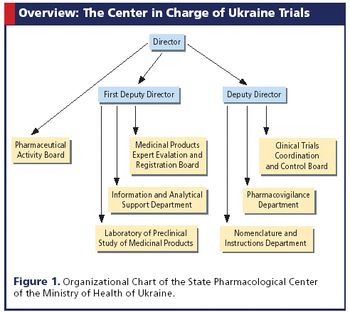
Trials are on the rise in the country, where recruitment rates are high but custom procedures can pose a problem.

Applied Clinical Trials
Lack of regulatory cohesion across member states a source of frustration for many in industry.

Applied Clinical Trials
Data JumpStart helps companies deploy standardization and conversion solutions