
Applied Clinical Trials
BMS executive shares his perspectives on the current biomarker landscape, the role of translational medicine in accelerating this area of study, and how BMS is working to advance biomarker research across their R&D portfolio.

Applied Clinical Trials
BMS executive shares his perspectives on the current biomarker landscape, the role of translational medicine in accelerating this area of study, and how BMS is working to advance biomarker research across their R&D portfolio.

Applied Clinical Trials
Jill Wechsler interviews FDA Commissioner Scott Gottlieb about his push for more efficient R&D to help lower drug prices.

Applied Clinical Trials
Peter O’Donnell explores potential parallels of the U.S. “right-to-try” debate in Europe.

Applied Clinical Trials
May includes a number of events to get more visibility for clinical trial awareness, and to offer it as a care option.

Applied Clinical Trials
Examining the unique standards and related challenges when assessing the safety and efficacy of cancer immunotherapy candidates.
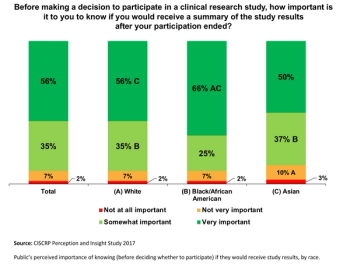
Applied Clinical Trials
The third in a series of results from the Center for Information and Study on Clinical Research Participation’s (CISCRP) landmark 2017 Perceptions & Insights Study.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials May 2018 issue in an interactive PDF format.

Applied Clinical Trials
Though digital technology has improved the R&D process, when it comes to important clinical benefits and outcomes, modern biotech products have rarely shown the advantages of older generations of drugs.

Applied Clinical Trials
With technology’s increasing ability to gather and analyze previously unmanageable data sets, and medicine’s forays into genomics and targeted therapies, the time of the master protocol may be at hand.