
Applied Clinical Trials
New standards and research methods aim to provide more useful information on population subgroups.

Applied Clinical Trials
New standards and research methods aim to provide more useful information on population subgroups.

Applied Clinical Trials
Dismay over the level of anti-pharma feeling in the EU is not just "crying wolf."

Applied Clinical Trials
Move away from the "service" model if it means being subservient.

Applied Clinical Trials
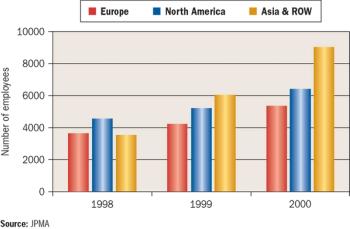
Long overlooked as global drug innovators, Japanese pharmaceutical companies are gaining attention for their novel clinical development programs.

Applied Clinical Trials
Well-planned studies designed to pinpoint additional data are increasingly important as a result of the EU's emphasis on added therapeutic value.

Applied Clinical Trials
A new, standard-based approach minimizes the delays between discovery of innovative agents and identification of their therapeutic benefit for patients-critical in cancer research.

Applied Clinical Trials
Arthur L. Caplan, Ph.D., chair of the University of Pennsylvania's Department of Medical Ethics, expressed his frank views about issues that are ailing the drug industry today during his DIA Keynote Address in mid-June.