With more clinical trials conducted overseas, sponsors are facing new obstacles.
Faiz Kermani, PhD, is a budgets, proposals, and marketing executive with Chiltern International Limited, a CRO in Slough, Berkshire, UK.

With more clinical trials conducted overseas, sponsors are facing new obstacles.

CSR is in the best interest of the public at large...and the pharmaceutical company itself.
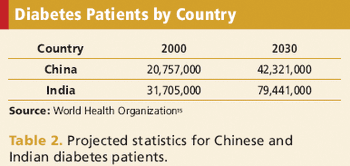
This Asian market holds great potential for the future, including possible collaborations with global pharma partners.
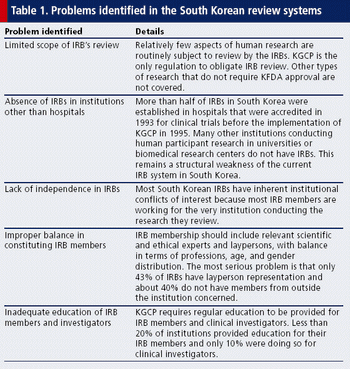
The Declaration of Helsinki requires that control groups receive the “best” current treatment, not the “local” one. This shift in wording has profound implications.
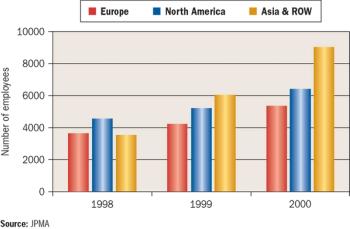
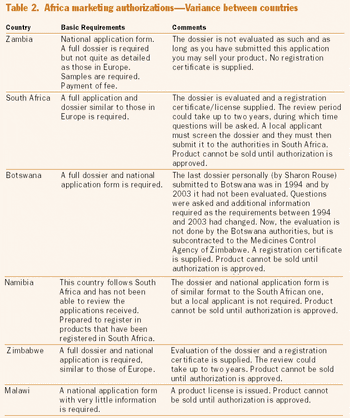
Long overlooked as global drug innovators, Japanese pharmaceutical companies are gaining attention for their novel clinical development programs.

To minimize time to market, sponsors must explore new subject recruitment techniques.

Published: June 1st 2011 | Updated:
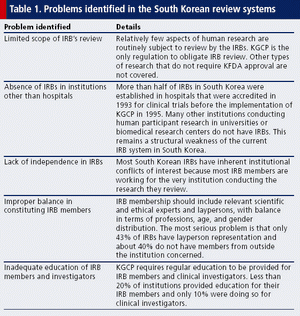
Published: November 1st 2005 | Updated:

Published: May 1st 2006 | Updated:
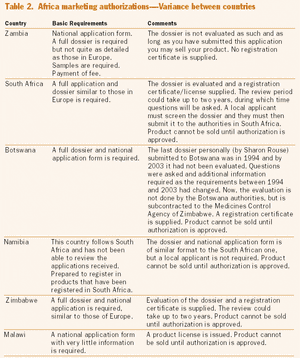
Published: March 1st 2005 | Updated:
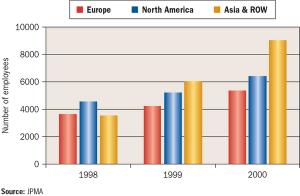
Published: August 1st 2004 | Updated:

Published: February 1st 2003 | Updated: