
Applied Clinical Trials
2011 Directory & Buyers Guide Services & Products Company Index

Applied Clinical Trials
2011 Directory & Buyers Guide Services & Products Company Index

Applied Clinical Trials
CRO/SPONSOR : Clinical Contracting Efficiency Can Save Millions REGULATORY : Common Technical Document Development - A Partnership Approach Also in this issue : FDA Looks to Collaborate Overseas, EU Reviews, Clinical Trial Rules, Meaningful Web, Greater Trial Insight

Applied Clinical Trials
A meeting in July to discuss changes to the old rules explored the need for compromise across the industry.

Applied Clinical Trials
The EFGCP annual conference in January aims to improve information and empower patients.

Applied Clinical Trials
The positives of "going digital" are becoming more and more apparent for clinical research.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
Patient-facing clinical trials are finally implemented a decade later.

Applied Clinical Trials
Pharmaceutical testing and production overseas spurs call for more reliance on counterparts.

Applied Clinical Trials
Knowledge is power, but power is of no value if you don't exercise it.

Applied Clinical Trials
A simple but overlooked way to save millions of dollars during new drug development.
Applied Clinical Trials
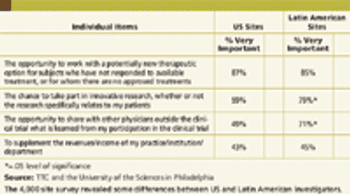
Starting with Eastern European sites in the 1990s, companies have increasingly selected investigator sites outside of North America and Western Europe.

Applied Clinical Trials
Partnership formation is the new trend in common technical document development.