
Applied Clinical Trials
REGULATORY : Document Management Practices CARDIAC SAFETY : Safety Testing Has Evolved Also in this issue : PDUFA Legislation Expansion, EU Pharmacovigilance, Aggregate Data, Sunshine Act

Applied Clinical Trials
REGULATORY : Document Management Practices CARDIAC SAFETY : Safety Testing Has Evolved Also in this issue : PDUFA Legislation Expansion, EU Pharmacovigilance, Aggregate Data, Sunshine Act

Applied Clinical Trials
Drug, biological supply, and medical device manufacturers must track all payments of over $10.

Applied Clinical Trials
Testing cardiac safety has developed into more than measuring a simple ECG test.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
UK officials stress importance of evaluating benefits of new therapies before approval.

Applied Clinical Trials
Public to private switch gives CROs better chance to "fix" and transform themselves.

Applied Clinical Trials
Five key steps for e-submission ready documents to avoid pre-submission rework.

Applied Clinical Trials
New ClinCard system helps deliver payments in a faster and more efficient manner.

Applied Clinical Trials
Multiple regulatory and reimbursement proposals are slated to expand legislation to renew PDUFA.

Applied Clinical Trials
EU's new rules will be influenced by more than just those primarily responsible for pharmaceuticals.
Applied Clinical Trials
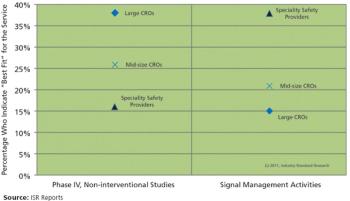
Industry Standard Research has recently published new research titled "Post-marketing Safety Market Dynamics," which deeply dives into the growth and decline of study conduct and outsourcing in the post-marketing safety arena.

Applied Clinical Trials
Cornerstone Pharmaceuticals' CEO Robert Shorr discusses oncology drug development challenges.