Applied Clinical Trials
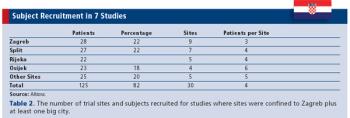
GLOBAL TRIALS : South Africa?s Positioning Fosters Healthy Trial Landscape Croatia?s Dynamic Qualities CROs Must Manage Italy?s Regulatory Makeover EDC?s Evolution in Japan Also in this issue : Postmarketing Pushes Back Approvals, EU Regulators Increase Involvement, Site Shake-Out Benefits Those That Remain, Is in Silico Modeling the Economic Solution?