
A Greenphire study of 600 clinical trial professionals from various stakeholders weigh in on emerging patient engagement trends

A Greenphire study of 600 clinical trial professionals from various stakeholders weigh in on emerging patient engagement trends

Patients will now have more options for how they receive their expense or stipend payments related to clinical trial participation.

Analysis of 13,490 clinical trials finds three of the top five most-studied diseases fall within oncology.

In an interview with Applied Clinical Trials' sister publication, Pharmaceutical Executive, Elizabeth (Beth) Garner, chief scientific officer for Ferring Pharmaceuticals, discusses what it was like transitioning from working as a gynecologic oncologist and seeing patients to working in pharma, specifically clinical trials.

Expansion aims to continue to create programs that address health and education inequities.

WCG publishes report providing assessment of motivators across all clinical research stakeholders, including patients, sites and sponsors.

GxP Inventory available in two options for varying sponsor and CRO requirements.

Privia Care Center locations that do not currently conduct clinical trials will receive embedded research from Velocity.

The confirmatory study—which tracked 18 months of data—helped clear the way for new Alzheimer’s drug.

Union adds to company’s goal of reaching net zero emissions by 2045.

Real-world evidence solutions provider will join Thermo’s Laboratory Products and Biopharma Services segment.

Devana's workflow and analytics solutions will be integrated into RealTime's Site Operations Management System.

Jeff Liter, CEO of Luminary Therapeutics discusses the company's unique approach in the fast-evolving landscape of cancer treatment.

IgniteData will provide data transfer integration between the EHR at MSK and the electronic systems of two major clinical trial sponsors.
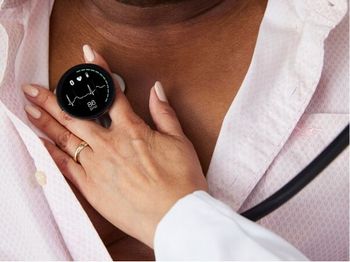
Eko Health developed the device, which has received 501(k) clearance from the FDA.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Venkatraman Balasubramanian, SVP and global head, industry solutions, healthcare and life sciences, Orion Innovation, discusses feedback from the audience during his session, “The Case for Generating Synthetic Data as Real-World Data: Regulatory and Planning Perspectives.”

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Venkatraman Balasubramanian, SVP and global head, industry solutions, healthcare and life sciences, Orion Innovation, provides a synopsis of "“The Case for Generating Synthetic Data as Real-World Data: Regulatory and Planning Perspectives.”

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Courtney Granville, Director, Scientific Affairs, Drug Information Association, discusses issues that will be addressed at the upcoming FDA town hall.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Courtney Granville, Director, Scientific Affairs, Drug Information Association, discusses analyzing AI in Regulatory Decision Making

Partnership will support imaging in oncology clinical trials.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Tamei Elliott, Senior Manager, Scientific Programs, Drug Information Association, discussed inroads in clinical trials for underserved populations.

At-home blood sample collection and analysis solutions for post-approval research through joint offering.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Tamei Elliott, Senior Manager, Scientific Programs, Drug Information Association, discusses DIA educational opportunities improving clinical operations staff on DE&I concerns.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Tamei Elliott,Senior Manager, Scientific Programs, Drug Information Association, discusses changes to the role of diversity in clinical trials in regard to scientific programing.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Cal Collins, CEO of OpenClinica discusses the near-term and long-term future of SMART on FHIR.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Cal Collins, CEO of OpenClinica discusses the current state of SMART on FHIR and its impact on the usefulness of real-world data.

In an interview at the DIA Global Annual Meeting with Nicholas Saraceno, Cal Collins, CEO of OpenClinica discusses his passion for SMART on FHIR.

Integrated eCOA & ePRO solution taps company’s technology expertise.

DIA session offers an overview of the current regulatory landscape and challenges for both sponsors and regulators in achieving quality and integrity in the fast-moving area.
Collaboration to focus on digital vascular biomarker patient monitoring trial.