
Industry experts share their thoughts on the evolution of the functional service provider model, as demand for flexible resourcing grows.

Industry experts share their thoughts on the evolution of the functional service provider model, as demand for flexible resourcing grows.

Stakes are high for emerging biopharmaceutical companies to get their relationships with CRO partners right from the outset.

Biometric functional services, more than any other, can have a significant impact through the standardization of data collection and management.

Creative approaches are needed to address the clinical research workforce “talent wars”

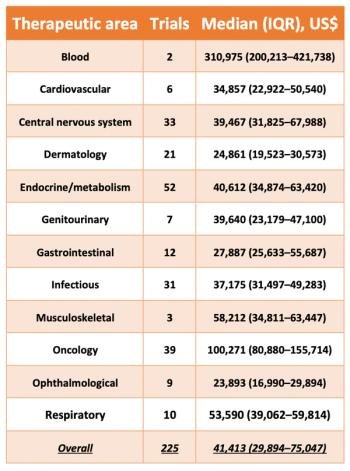
The Institute for Safe Medication Practices has released a new study which breaks down the estimated costs associated with the approval of new pharmaceutical treatments.

Excerpts resulting from the extended discussion on CRO oversight at the recent CBI Finance and Accounting for Bioscience Companies conference.

In this interview, Linda Rawlings, VP of Neurodegenerative Development at Synteract, elaborates on addressing the challenges of neurodegenerative disease clinical trials.

Joe Pollarine, Head of GxP Systems Strategy Director at Janssen, recently spoke about CRO oversight models and will expand on these models in this interview.

As the size, complexity, duration, cost, and globalization of clinical trials has grown, pharmaceutical and biotech companies have moved to outsource clinical activities to CROs to achieve a wide range of objectives.

There are key cultural differences to be aware of before conducting clinical trials in Japan.

Proposed partnership model explores the value of establishing alliances between CROs and networks of small and emerging biopharma companies.

Exploring sourcing model options for appropriate incorporation of DMCs into a clinical program.
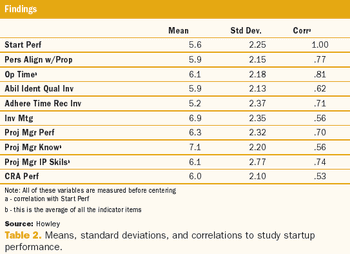
A two-phase statistical analysis identifies the key performance drivers in clinical trial startup.

MAPs can effectively address unmet patient needs and become a cornerstone of product strategy.

Pharma needs to find a balance in working with Sponsors and CROs in order to streamline the study processes.

Clinical research organizations should look to peer-to-peer mentoring for professional development.

Companies trusting each other and looking out for each other's interests is the hallmark of outsourcing.

The goal is to have an evidence development framework that can answer a range of questions simultaneously.
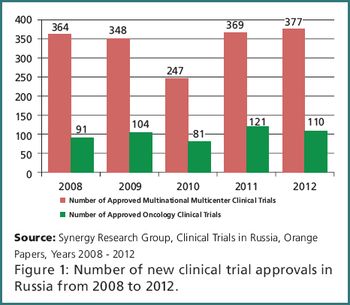
Oncology clinical trials: A case for Russia and Eastern Europe.
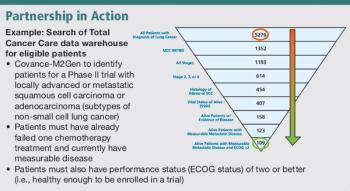
Accelerating the discovery and delivery of personalized medicine.

Increasing development costs and high failure rates require earlier integration of imaging data in the Phase I setting.

Life can change in an instant, but there is one thing that always abides: hope.
Options have emerged that make DIY EDC technology more accessible to smaller organizations.
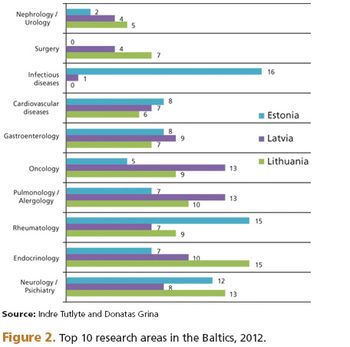
The region has an appropriate population size, solid infrastructure, experienced nvestigators, and short timelines.