
CRO/Sponsor
Latest News
Latest Videos

More News

How pharmaceutical start-ups can mitigate risk and avoid costly hiring mistakes.

Industry provides endless methods in crafting solutions for patients.

Finalizing protocols, aligning teams, and staying engaged headline best practices.

Reminding clinical research stakeholders to reprioritize routine assessment of patient satisfaction as a primary outcome measure in patient-centric activities.

How CROs have adapted to recent challenges—and how they’ll continue to evolve in the future.

Brief history lesson sets stage for current state of CROs.

Industry must take advantage of "lessons learned."

Characterizing the long-term adoption experience of clinical technologies and capabilities—and what senior leaders can do to drive adoption within their walls.

Stakeholders from across the industry must discuss ownership to ensure effective implementation.

Examining best practices in using the functional service provider model for clinical operations, pharmacovigilance, regulatory, and other areas.

Proactive, pervasive, and strategic planning lead industry into future.

In this interview, Dave Hanaman, co-founder, president and chief commercial operator at Curavit Clinical Research discusses launching the organization at the start of the pandemic and how it operates with a commitment to designing trials with a fully virtual site.

Strong collaboration critical as trial development advances.

Technology, talent and specialization add up to a plethora of industry M&As.

A move back to in-house management of clinops could be on the horizon for sponsors.

A brief look at the market for mergers and acquisitions since the year of COVID.

New BDO CRO Insights Report reveals high rate of CRA turnover and what organizations are doing to combat it.

Steven Bukvic, CEO of the ACROSS Global Alliance, speaks to Applied Clinical Trials about how it works to improve the clinical trial experience for smaller- and medium-sized sponsors.

The drug development sector is embracing technologies and digital methods that were previously not as widely used due to the COVID-19 global health crisis.

Nearing a year into the pandemic, this article describes the legal concerns CRM encountered during the COVID-19 outbreak.
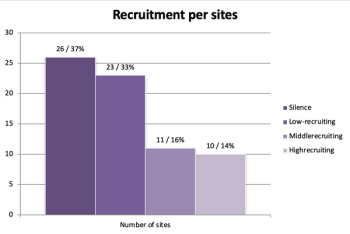
The success of a clinical study depends on the possibilities to involve the patient in the study.

FDA’s Vaccines and Related Biological Products Advisory Committee addresses issues related to testing and approval of potential COVID vaccines.
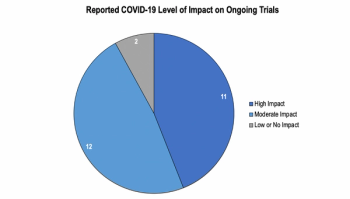
Findings from a Tufts study examining the effects of COVID-19 on clinical trials.

Recognizing pre-pandemic pain points, such as patient engagement and protocol development, could lead to post-pandemic trial success.

Evaluating the lessons learned about trial sites from COVID-19 to form new strategies and improve patient safety for the future.


