
A well designed CTCP can keep everyone motivated and focused on achieving trial objectives.

A well designed CTCP can keep everyone motivated and focused on achieving trial objectives.
The major industry milestones of the past 20 years.

The adoption of new, consistent definitions are needed to help demystify late phase trials.
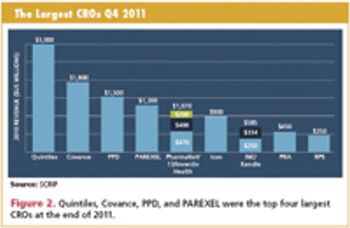
Continual change is coming as sponsors' sourcing requirements diverge from the major CROs.

Where do CRO's turn next? What does pharma do about it?

Cancer trial recruitment is struggling-adult participation is limited.

Optimizing early phase trials key to the success of novel cytotoxics and targeted therapies.

Are emerging markets the only answer to oncology clinical trial recruitment?

Where do CRO's turn next? What does pharma do about it?
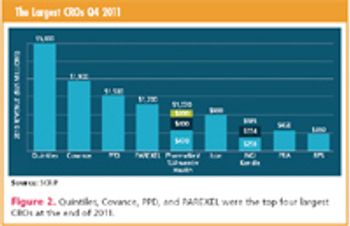
Continual change is coming as sponsors' sourcing requirements diverge from the major CROs.

Researchers who work on HIV vaccines face a major challenge: convincing members of at-risk communities to volunteer for these trials.

Partnering with CROs and using a blinded independent central review can increase trial success.
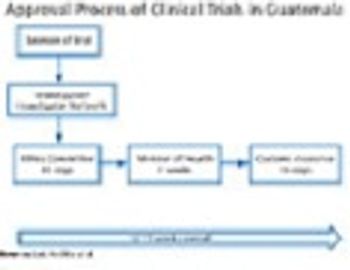
Guatemala is multi-ethnic, multilingual, and multi-cultural-ideal for international trials.

Research shows four factors best predict successful patient enrollment in clinical trials.

BRIC governements are working to make it easier to conduct trials in country.

Drug, biological supply, and medical device manufacturers must track all payments of over $10.

Knowledge is power, but power is of no value if you don't exercise it.

A simple but overlooked way to save millions of dollars during new drug development.
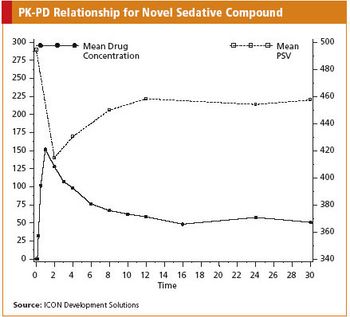
Two unique techniques help characterize pharmacodynamic drug effects in healthy subjects.

Collaborative communities may hold the key to transforming a half-century's old R&D paradigm.

The proper storage of research documents remains an essential aspect of the clinical trials process.

Transfer of material between the supply cahins can be time consuming and complicated
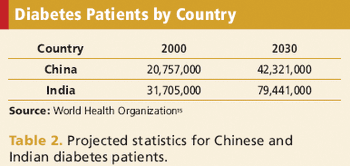
With more clinical trials conducted overseas, sponsors are facing new obstacles.

Creating a drug's safety profile through the use of benefit-risk assessments during development.

Medicine is not quantum mechanics where we cannot predict when an event will occur