
While not a panacea, pharmacogenomics is still a valuable trial tool that can make the recruitment process more efficient and eliminate the high costs associated with late-stage product failure.

While not a panacea, pharmacogenomics is still a valuable trial tool that can make the recruitment process more efficient and eliminate the high costs associated with late-stage product failure.
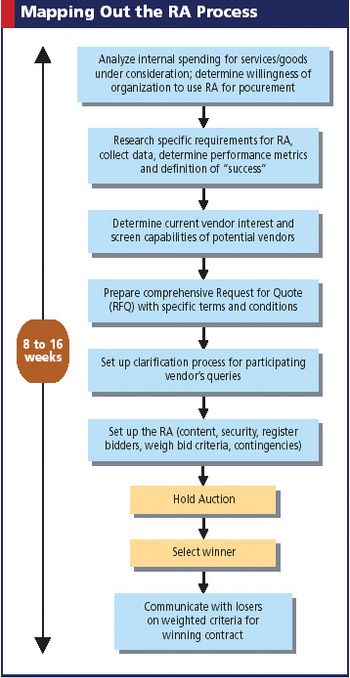
In this Web-based strategy, suppliers bid "down" prices for the privilege to sell their products and services. Is big pharma sold?

Generalizing fastest drug development strategies and practices.

Key screening, training, and communications techniques for putting together a strong staff.
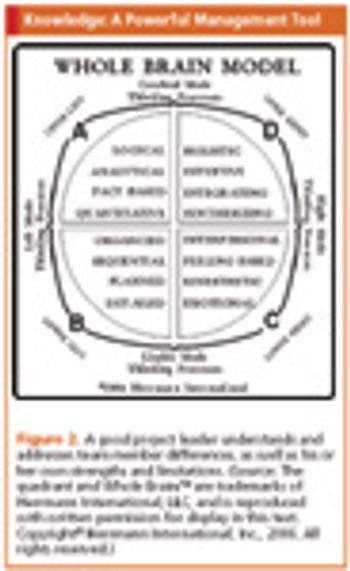
A review of the essential requirements for good project leaders and the crucial role they must play.

Boehringer International implemented a holistic approach to electronic data capture, boosting quality and efficiency.
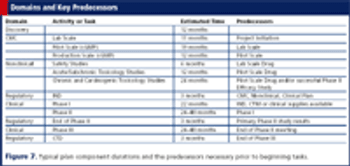
Using a template both accelerates a project's timeline and infuses it with best practices.

A collaborative model for increasing drug availability in resource-poor, disease-endemic countries
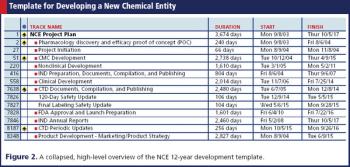
A project plan based on a fully integrated template can position senior management's expectations to minimize surprises.

Right now the industry has a chance to reclaim its good name and recapture the public's respect for the power of what it can do.

Rethinking the current pharmaceutical model

A single, industry-wide standard could streamline the clinical trial information management process.

With outsourcing on the rise, it's time companies reevaluated the role of CROs.

CSR is in the best interest of the public at large...and the pharmaceutical company itself.

Effective strategies for ensuring the long-term integrity of specialized biological samples
What to look for when outsourcing cardiac safety studies, including a compatible business mindset.
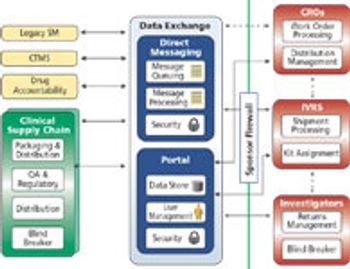
Some companies may be stretching their ERP systems and squandering opportunities for productivity gains.

The European pharma industry struggles to regain its former prowess in R&D.

To clear a pipeline bottleneck, this Sponsor and CRO worked together as a single team.

Wyeth's positive experience with partnerships is a good example of R&D progress for the industry.

A growing trend in the industry has many pharmaceutical companies looking to manage the changing mix of global clinical trial locations.
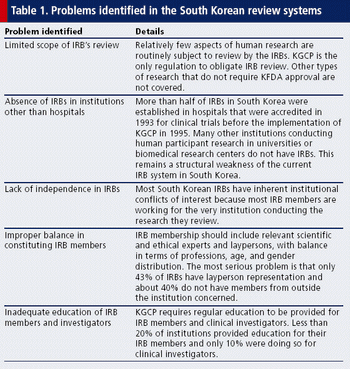
This Asian market holds great potential for the future, including possible collaborations with global pharma partners.

Much has been written about the staggering costs of drug development and how the low Phase III success rates across the pharma industry have contributed to these costs. While safety outcomes explain many failures during the early development phase and have likewise played a prominent role in some highly publicized product withdrawals, efficacy failures in Phase III have received little attention. What we now know, however, is that a significant number of Phase III failures are attributable neither to issues of safety nor product differentiation, but to an inability to confirm efficacy against placebo.
This innovative method increases flexibility, saves money, and supports the Critical Path Initiative.
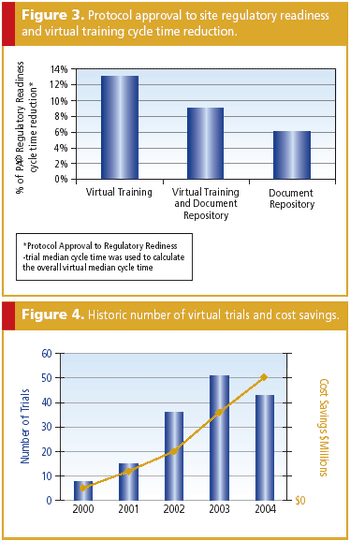
Virtual tools and processes are a key way to decrease costs while increasing quality.