
Tech companies entering space must adapt and adhere to regulations set by FDA.
President, CEO, THI Pharma Services Inc.

Tech companies entering space must adapt and adhere to regulations set by FDA.

Brief history lesson sets stage for current state of CROs.

New requirements must be put in place to ensure data quality and integrity.

Lessons learned from FDA’s current process and proposed alternative review strategies.
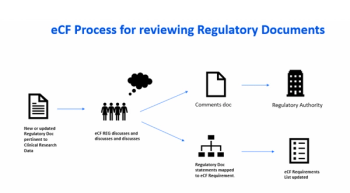

How to assure that software used in clinical trials will support regulatory scrutiny during pre-approval inspections and application review.

When designing and using an electronic informed consent form, there are many factors that should be considered when applying to clinical research.

The time is now for sites and sponsors to put away fears associated with data acquisition and monitoring technologies-and better support the regulatory push in recent years for standardized adoption of paperless trials.

The need to involve regulators is crucial when the use of electronic data devices impacts the management of patient safety and evaluation of trial endpoints.

How to satisfy regulatory concerns about EDC data integrity and site controls over its source records.

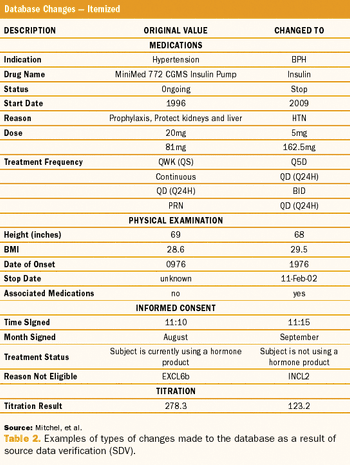
Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.

The eClinical Forum Risk Based Monitoring Taskforce offers some best practices for ensuring clinical data quality.
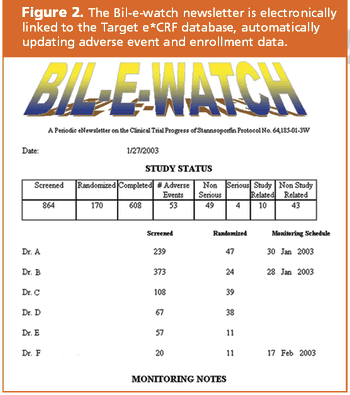
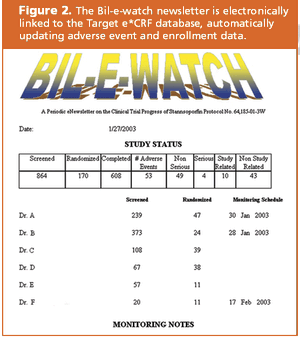
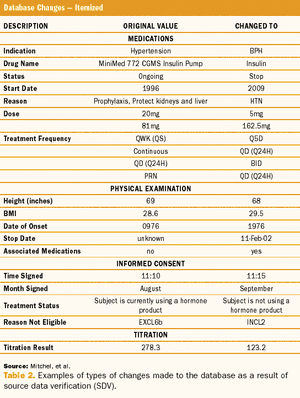
Target Health and Infacare used a CRF database to answer all of FDA's questions.

Published: March 10th 2022 | Updated:

Published: August 1st 2013 | Updated:
Published: June 1st 2005 | Updated:
Published: June 1st 2014 | Updated:

Published: April 16th 2015 | Updated:

Published: August 1st 2015 | Updated: