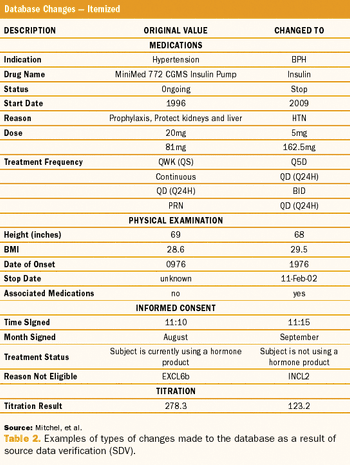
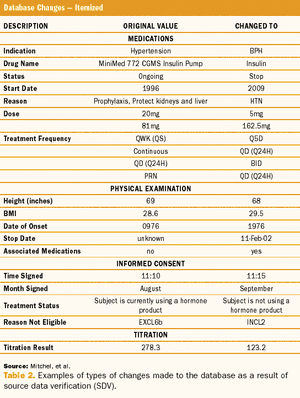
Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.
(Sergio.DallaNora@ferring.com) Associate Director of Clinical Research Ferring Canada

Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.
Published: June 1st 2014 | Updated: