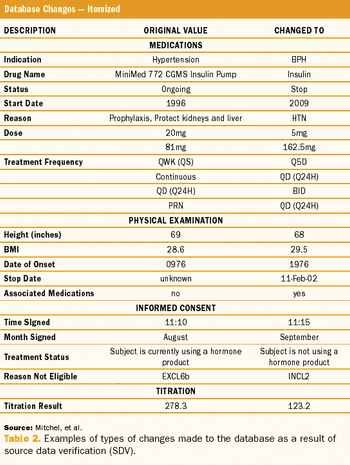
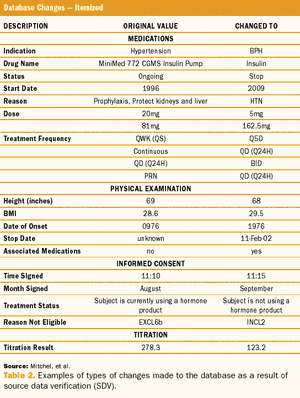
Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.
MS, Senior Project Manager (jmarkowitz@targethealth.com

Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.
Published: June 1st 2014 | Updated: