Cinzia Piccini
Advertisement
Articles by Cinzia Piccini

Advertisement
Latest Updated Articles
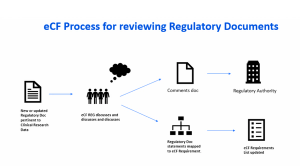 Keeping Up with the Regulatory Expectations
Keeping Up with the Regulatory ExpectationsPublished: July 8th 2019 | Updated:
Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
Accelerate Clinical Trials with AI-Enhanced Financial Management
4
SCOPE Summit 2026 Keynote Fireside Chat: Aligning Purpose, Innovation, and Operational Excellence in Clinical Development
5
