
New requirements must be put in place to ensure data quality and integrity.

New requirements must be put in place to ensure data quality and integrity.

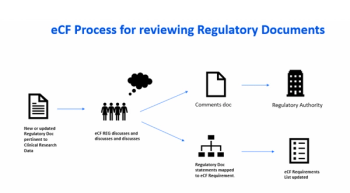
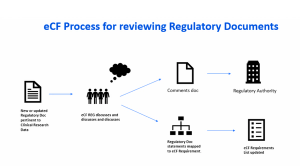
A discussion of the regulatory history behind audit trails and its interpretation as it relates to clinical research data originating or residing in EDC or eCOA systems.
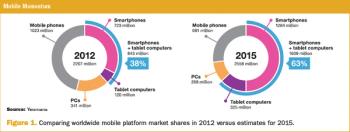
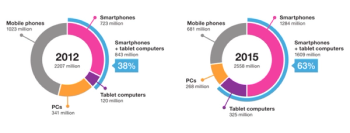
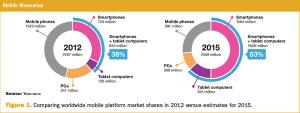
It is tempting to imagine the use of the patient's own mobile computing platform for collection of patient-reported outcomes (PROs).
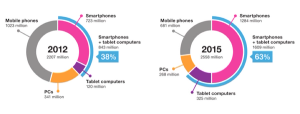
It is tempting to imagine the use of the patient's own mobile computing platform for collection of patient reported outcomes.
Published: January 28th 2014 | Updated:
Published: December 1st 2014 | Updated:
Published: July 8th 2019 | Updated:

Published: January 3rd 2022 | Updated: