
Lessons learned from FDA’s current process and proposed alternative review strategies.
Faculty, Program Coordinator, Senior Lecturer, MS in Regulatory Science & Food Safety Regulation Programs, Johns Hopkins University

Lessons learned from FDA’s current process and proposed alternative review strategies.
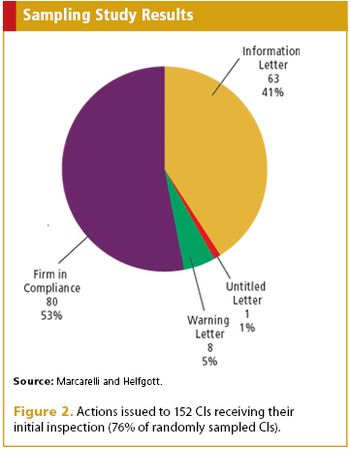
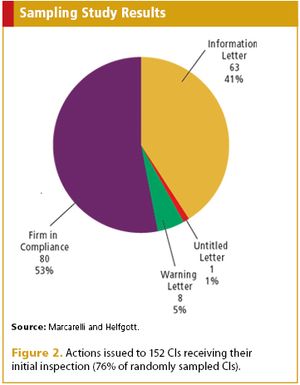
A probability sampling assessment by FDA takes a look at compliance in the medical device world.
Published: June 1st 2009 | Updated: