
The public considers clinical research important, but they're not fans of the people behind it.

The public considers clinical research important, but they're not fans of the people behind it.

Can the historical industry and vendor service relationship change to one where both parties benefit?
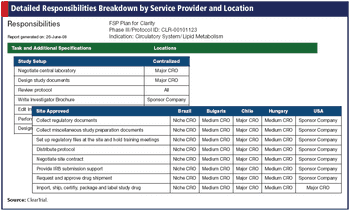
Outsourcing fundamentals for this pick-and-choose model so that both sponsors and service providers win.
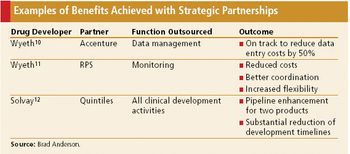
Productive relationships with CROs require a well thought out strategy on the part of sponsors.
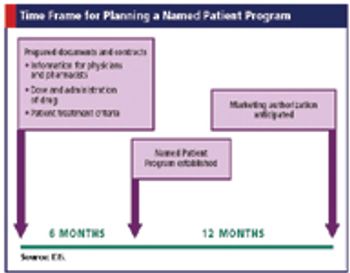
Integrating clinical trials and named patient programs to provide global access to drugs before approval.

Norbert Clemens, MD, head of clinical development for CRS Clinical Research Services, explains that EDC is more than just technology; it is indeed the new way of thinking about clinical research.

How leveraging local expertise can make the migration east smoother for companies.

Outsourcing finance and accounting can provide cost savings and improve operations.

A recent survey uncovers key criteria that influence a sponsor's decision when selecting a CRO.

Today's sponsors are waking up to the fact that changes made between phases can positively impact R&D.

Peek into a future where drugs are approved faster and R&D is less costly thanks to technology.
Performance and noncompliance clauses change the partnership between sponsor and vendor.

Readers respond to the NEJM article mentioned in November's From the Editor

Strategies for labs to support global trials and meet the needs of sponsors worldwide.

A look at ECG evaluation for drug-related alterations in repolarization and Torsade de Pointes.

To select the right CRO as an imaging partner, sponsors must first be aware of what to look for.

With globalization of trials comes the difficulties of biologic sample logistics. Enter the centralized/decentralized laboratory model.

How imaging CROs minimize data variability in multisite studies and create the necessary consistency.

Big pharma collaborates on routine audits and quality management of third-party service providers.
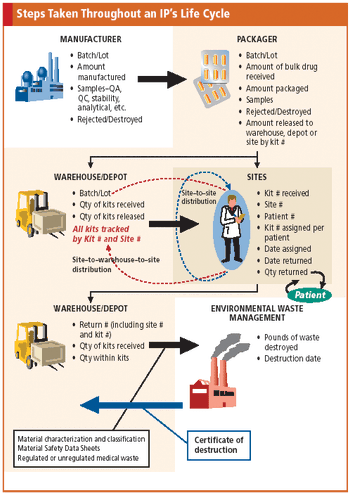
Tracking the life cycle of a drug is a complex process that early planning and IVR technology can help ease.

HemoBioTech Engages CRO to Facilitate Clinical Study in India of its Human Blood Substitute, HemoTech

New alternatives for e-clinical and data management. As EDC becomes more widely used in clinical trials, sponsors are not limited to one option but rather can choose different routes to optimize EDC efficiency.
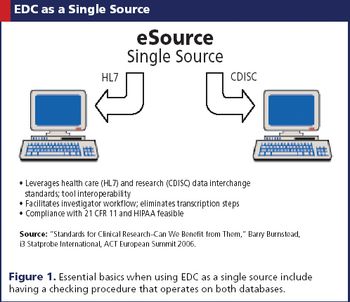
EDC is being embraced by both continents, where its efficiency is welcomed despite a few kinks.
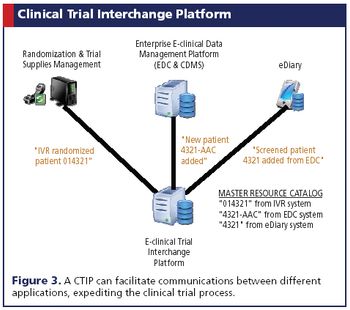
Emerging technologies are moving the industry closer to true IT solutions harmonization.

EDC is being embraced by both continents, where its efficiency is welcomed despite a few kinks.