
How different can the health care environments be from where data is derived to justify pooling?

How different can the health care environments be from where data is derived to justify pooling?

Why models such as BRIDG are essential in developing clinical research processes and applications

Integration with other systems such as electronic trial management systems is EDC's future.
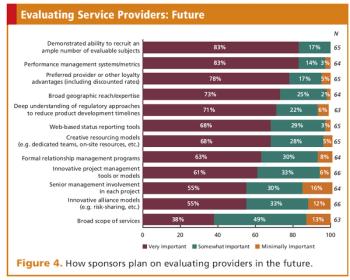
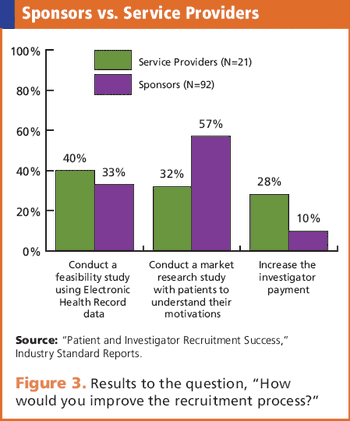
In-depth survey reveals trends, outlooks, and future plans of both sponsors and service providers.
The benefits of peer-to-peer networking over email, fax, FTP, and hosted solutions.

Sanofi Pasteur collects quality of life and safety data with eDiaries.
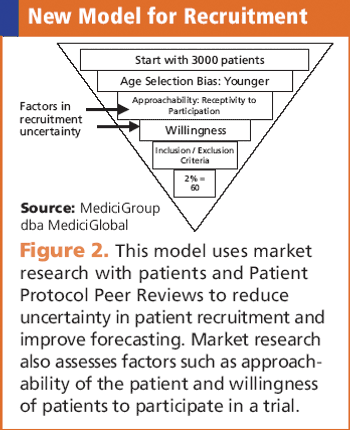
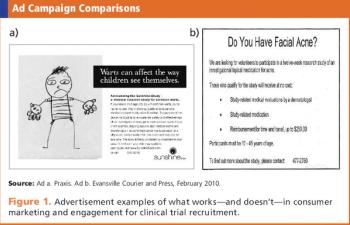
Clinical teams need to learn what motivates a patient to become a study subject.

The use of text messaging technology for recruitment in Phase I studies.
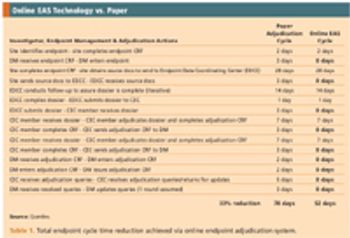
How an integrated, EDC-based system can optimize the endpoint process.

With clinical trials now a global industry, "back" translation requires a closer look.

Spending on research exceeds $35 billion, and clinical grant spending now tops $11 billion.

No one involved in clinical research is ever quite satisfied with the status quo.
There is a misalignment of experiences, expectations, and beliefs among subjects, investigators, sponsors, and service providers.
Systemized knowledge: experience, insight, and predictive modeling deliver successful enrollment.

Through digital signage, sponsors hope to increase enrollment in trials.

An intelligence-driven end-to-end approach defines new recruitment methods.

Promising data warehousing systems like Janus are still waiting to realize their potential.
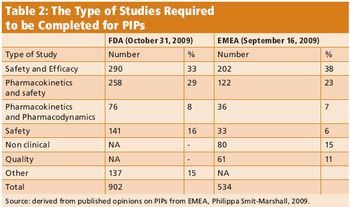
Global research in children affects industry and the trial environment.

Plan ahead for many seasonal diseases that could derail even the best laid clinical trials.
While it has yet to be fully integrated, electronic data capture is gaining ground in Japan.

Economic factors and sponsor practices will dramatically alter the investigative site landscape.
Economical development and regulatory standards make the country a logical choice for trials.
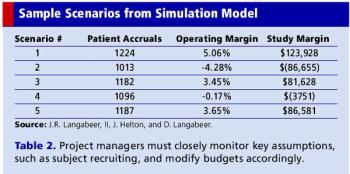
With a PC and some software you can generate realistic projections that save time and money.

The once recession-proof industry must now seek out opportunities to defend itself from potential downfalls.

Evolving out of disconnected technology solutions and working toward integrated processes.