Knowing what you want from a CROs proposal will help make the most of the selection process.

Knowing what you want from a CROs proposal will help make the most of the selection process.

With so much depending on accurate data, taking a do-it-yourself approach to scoring can be risky.
An integrated product development plan designed early increases the odds of on time success.
Improving the quality of radiological measurements in oncology trials with IAs.

Out of necessity, providers may finally become valued strategic partners.
Effective ways for sponsors to reduce risk when embarking on comparative clinical trials.
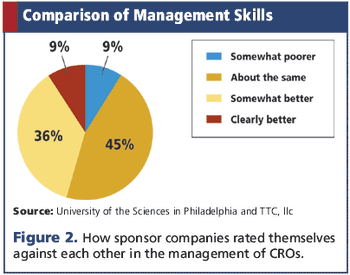
Study reveals how sponsors rate their own outsourcing abilities compared to other companies.

A more transparent solution to vendor selection in an evolving technology environment.

Two case studies illustrate the potential to turn chaos into meaningful clinical study reports.

Similarities exist, but successful design requires that CROs understand the differences.

What it takes to get all stakeholders on the same page and keep project timelimes on track.

Designing and adapting the latest technology to meet clinical trial needs and redeem the best of its multiple benefits.

Drug development, largely immune to past economic downturns, now faces a different climate.
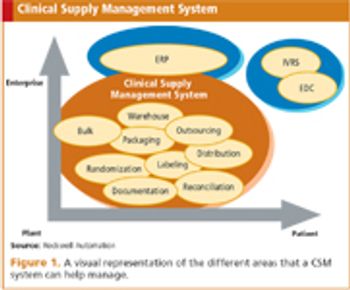
Improving the quality and speed of drug development through integration and interoperability.

Global clinical trial landscape changes pose new challenges for all sites-even AMCs.

How the merging of medical faculty and academic hospitals in The Netherlands is improving the country's research infrastructure.

Perspectives on adoption of technology at U.S. Academic Medical Centers and how sponsors will be impacted.

Can processes used for 20 years in the manufacturing and retail industries work for us too?

How one lab is using continuous process improvement to decrease timelines and redefine itself.

A look at the vague but time consuming requirements imposed on sites and efforts to ease the burden.

As patient recruiters and sponsors face low recruitment numbers, they must look beyond global trials to find a solution.
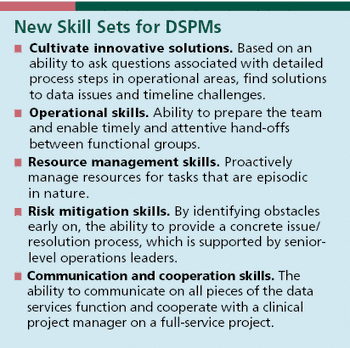
DSPMs offer a new skill set to drive data-heavy clinical trials and meet the needs of an evolving landscape.
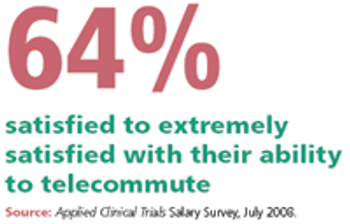
A look into the work-life benefits that are offered at a large contract research organization.
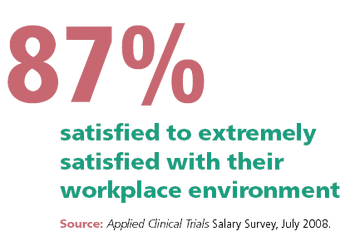
A look into the work-life benefits that are offered at a small contract research organization.

The need for collaboration between the various disciplines and stakeholders is becoming crucial.