
SLS009 is a novel CDK9 inhibitor under investigation for the treatment of relapsed/refractory peripheral T-cell lymphomas.

SLS009 is a novel CDK9 inhibitor under investigation for the treatment of relapsed/refractory peripheral T-cell lymphomas.

Exagamglogene autotemcel (exa-cel) has shown the potential to be a landmark therapy in preventing episodes of excruciating pain among patients with sickle cell disease.

The branded form of secukinumab is currently the only FDA-approved fully human biologic that directly inhibits interleukin-17A.

Abatacept is indicated across multiple inflammatory conditions, including for the treatment of adult patients with moderately to severely active rheumatoid arthritis, pediatric patients with moderately to severely active polyarticular juvenile idiopathic arthritis, and active juvenile psoriatic arthritis.

Furmonertinib is in development for the treatment of advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations.

The FDA granted Wezlana with interchangeable designation after clinical trials found no clinically significant differences in safety and efficacy for the indicated conditions across multiple inflammatory diseases.

The approval of pembrolizumab (Keytruda; Merck) combined with gemcitabine and cisplatin for the treatment locally advanced unresectable or metastatic biliary tract cancer is the sixth sixth indication for the anti-PD-1 therapy for gastrointestinal cancers.

Exploring the implications of FDA’s renewed focus and commitment to expand the use of accelerated approval for gene therapy products.

Probe targets agency’s role in advising on drug testing and submissions.

Measures being mulled to reform the AA process and address public concerns around its risk-benefit payoff.

Quality metrics, more domestic production aim to avoid supply disruptions and drug shortages.

The US Congress approved a federal spending package that increases funding for a range of programs to advance health and medicine.

FDA emphasizes importance of inclusion in trials.

This week's much-anticipated meeting of FDA’s vaccine advisory committee will address critical issues related to the testing and approval of vaccines to prevent COVID-19 infection.

Fears about overly accelerated development programs has heightened demands for wider access to information on study protocols, statistical analysis plans, and early results.

Shawn Singh, Chief Executive Officer of VistaGen Therapeutics, discusses the company's ongoing efforts to treat and help manage the anxiety symptoms related to the triggers and anxiety-related disorders COVID-19 provokes, and the FDA’s consensus on the design of their pivotal Phase III study of PH94B.

An amendment to Pediatric Research Equity Act, as part of the 2017 FDA Reauthorization Act, goes into effect soon aiming to change the landscape and promote pediatric cancer drug development.

A first vaccine against this coronavirus could still take some time to develop, but mRNA vaccine platforms could offer an early breakthrough.

An amendment to Pediatric Research Equity Act, as part of the 2017 FDA Reauthorization Act, goes into effect soon aiming to change the landscape and promote pediatric cancer drug development.

FDA's plans to resume inspections of some US regulated facilities this week are not clear on which sites will be visited and what alternative oversight strategies will apply where on-site inspections are difficult.

The need for treatments to combat the spread of COVID-19 is promoting greater cooperation among drug regulatory authorities around the world, with FDA officials communicating more frequently with their counterparts in Europe, Canada, Japan and other nations through established programs and agreements.

The research community is moving quickly to launch clinical trials of potential countermeasures, while regulatory authorities aim to support product development through regulatory flexibility.

FDA’s new commissioner, Stephen Hahn, announced his priorities at an FDA “all hands” staff meeting last week. Jill Wechsler reports.

While not setting any records for the rapid approval of new drugs in 2019, FDA did speed a number of important new therapies to patients, writes Jill Wechsler.
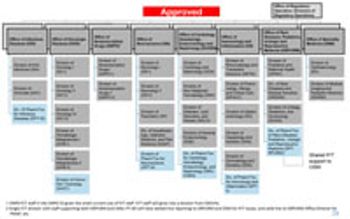
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.