
Claims of success for pharmacovigilance are under question, but hopes are higher for new clinical trials rules.

Claims of success for pharmacovigilance are under question, but hopes are higher for new clinical trials rules.
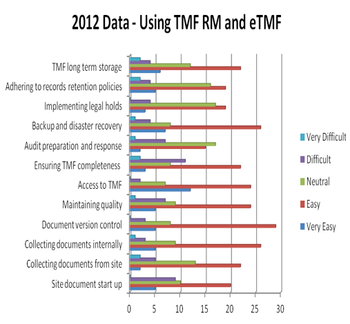
One of the many activities across the clinical development cycle that is non-negotiable is the creation, collection, management, and storage of the documents that are contained in the Trial Master File.

The advent of new drugs for tuberculosis is posing some interesting challenges for regulators in Europe.

Pharmaceutical companies are jumping on the clinical trial transparency bandwagon, while also seeking to protect confidential information.

The shift to personalized medicine has begun to account for a greater portion of new therapies in pharmaceutical pipelines, and the biomedical research community is watching to see if this trend continues in the coming months.

Global action and collaboration is needed to tackle the threat of antibiotic-resistant diseases.

Pharmacometrics optimizes the use of Phase II data to support Phase III success.

Compromise is going to play a key role as the clinical trial rules update debate rages on.

Will the clinical trials community assemble their arguments, and will they do it in time?

FDA commissioner Margaret Hamburg has formed a top-level working group to propose strategies for enhancing agency functions and processes, starting with the relationship between FDA Centers and its field force.

Industry organizations weigh in on the transparency debate.

The EMA is committed not just to greater accessibility of data, but proactive publication.
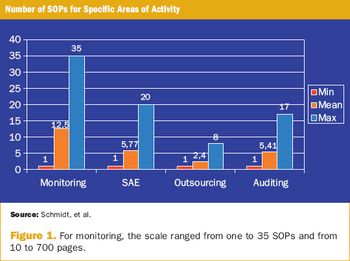
Survey appraises the use of SOPs in clinical research from the sponsors' point of view.

Women should be represented more in studies and greater attention paid to their general health issues.

We are now being encouraged by regulators worldwide to re-think our approach to monitoring and apply risk-based monitoring strategies rather than continue with our more traditional approaches.

The statistics show that fewer and fewer physicians are willing to take part in trials and that around half of those that do so once never do so again.

Debate rages on, European Parliament set to decide on new clinical trial rules in late October.

There are scarce or illdefined national and international regulations on quality control and standardization of biorepositories.

The clinical trial debate in the European Parliament is bogged down by rival schools of thought.

Consistent, effective process improvement can be the key to reducing delays and improving data accuracy.

European Commission's proposed system faces scrutiny from three separate committees.

Researchers from sub-Saharan countries are going to have better chances of upgrading their skills.

Decision makers can increase collaboration, transparency, efficiency, and effectiveness, while reducing development risks.

The EMA's predictions are a measure of optimism against a rather somber background.

Standard operating procedures need to be in place for the handling of suspected research misconduct.