
Some believe that the medical evidence base is distorted by missing clinical trial data.

Some believe that the medical evidence base is distorted by missing clinical trial data.

The European Medicines Agency responds to concerns over conflict of interest and openness.

PCORI, methods panel to set policies for comparing drugs and medical products and practices.

Pharmacists and patient groups welcome the new European Union directive on pharmacovigilance.

Routine inspections and regulations can help maintain GCP standards in global trials.

Reform law requires tracking and disclosure of fees to investigators and research consultants.

Waiving inclusion/exclusion criteria affects investigators, subjects, sponsors, and the trial itself.

The act creates serious concerns about the industry's ability to recruit and retain well-qualified investigators.

A new report from EFGCP and EUCROF suggests changes to geriatric trials.

A system of checks and examinations that helps ensure the quality of clinical trials.

The NICE will possess greater power, scope, and status under the new system.
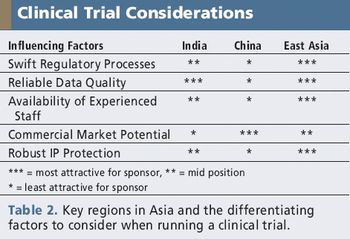
Rapid recruitment, potential cost savings, and investigative sites are just a few of the factors attracting sponsors to the region.

Sponsors, health care providers weigh pros and cons of REMS for bringing risky products to market.

FDA is saying that a study coordinator "generally" performs critical functions, such as subject recruitment.

Inspector General study focuses attention on quality of data and patient safeguards.

European network could bring confusion to health technology assessment and clinical research.

The European Medicines Agency has redesigned its Web site to improve transparency.

Personalized medicine enters the arena.

The verdict is awaited on the much criticized and long anticipated European clinical trials directive.

The 12-year data exclusivity period has significant implications in delaying the launch of biosimilars.

More information may be available on drug applications to expand public understanding of FDA policies.

The 12-year data exclusivity period has significant implications in delaying the launch of biosimilars.

New guidelines are being developed in Europe to address questions regarding stem-cell medicines.
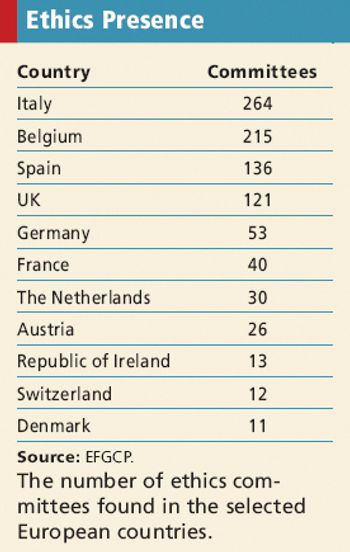
The newest report from the European Forum for Good Clinical Practice identifies the widely diverse ethical approval system across Europe.
Employing a three-component risk exclusion model in the assessment of investigational drugs.